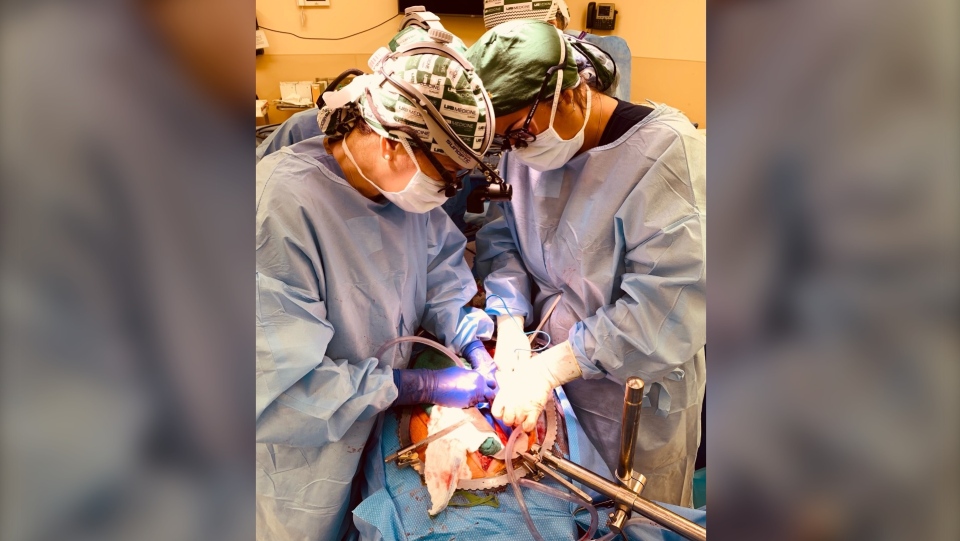
For the first time, scientists have successfully transplanted genetically modified pig kidneys into the body of a brain-dead human, an important step toward animal organs potentially becoming viable for transplants to save human lives in the future, something that could help address organ shortages worldwide.
On Thursday, researchers with the University of Alabama (UAB) published the first peer-reviewed study to document this process in depth.
The pig kidneys were taken from pigs that had been genetically modified with 10 key gene edits in order to make the kidneys capable of being transplanted into a human body.
After transplantation, researchers confirmed that the kidneys were not rejected and were able to filter blood and produce urine for the three days of the study.
"This game-changing moment in the history of medicine represents a paradigm shift and a major milestone in the field of xenotransplantation, which is arguably the best solution to the organ shortage crisis," Jayme Locke, director of the Comprehensive Transplant Institute in UAB’s Department of Surgery and lead surgeon for the study, said in a press release.
"We have bridged critical knowledge gaps and obtained the safety and feasibility data necessary to begin a clinical trial in living humans with end-stage kidney failure disease."
The hope is that the procedure researchers followed for the transplantation could establish a model to be followed in the future when performing a transplant of a pig kidney into a living human recipient.
Researchers are proposing that this procedure be called "The Parsons Model" after the donor who made this study possible.
THE RECIPIENT
Jim Parsons, 57, was a registered organ donor when an accident left him brain-dead.
"He was on a dirt bike doing what he loved most," Julie O’Hara, his ex-wife, explained. "His favourite activity in the world."
Parsons had been riding dirt bikes since he was a child, having participated in hundreds of races.
"He’d had many accidents before, and this was just a freak accident that took him from us," she said. "He was declared brain-dead, and I had requested to speak with someone about organ donation and to know what he was able to give."
But shortly before he was to undergo surgery to remove some of his organs for potential donation, O’Hara received a call, asking if she would speak to Dr. Locke about a study.
After the study was explained to them, they called a big family meeting to make sure everyone agreed.
"We all, unanimously, without a doubt, 100 per cent, is what we decided he would’ve wanted," she said.
His daughter Ally added that "there wasn’t any question."
His family agreed to let researchers keep Parsons on a ventilator in order to keep his body functioning so that researchers could attempt the transplant.
"Jim was a never-met-a-stranger kind of guy who could talk to anyone and had no enemies — none," O’Hara said.
"Jim would have wanted to save as many people as he could with his death, and if he knew he could potentially save thousands and thousands of people by doing this, he would have had no hesitation. Our dream is that no other person dies waiting for a kidney, and we know that Jim is very proud that his death could potentially bring so much hope to others."
THE POTENTIAL IMPACT
Kidney disease affects hundreds of thousands of people worldwide. According to the Canadian Institute for Health Information, in 2019, more than 23,000 Canadians were receiving dialysis treatments for chronic kidney failure.
Those on dialysis either have to remain on dialysis for the rest of their lives or receive a kidney transplant — but the waiting list can be years long.
Dr. Selwyn Vickers, dean of the School of Medicine at UAB, said this new research is significant not only for patients waiting for kidney transplants but for families who fear losing their loved ones while on a waiting list.
"There are thousands of people who need organ transplantations that are on a list and the amount of organs that are available are clearly limited," he said. "And there are a number of people who die waiting in that process."
Locke said that what this experiment allowed them to do was to really test feasibility and safety when it comes to transplanting a pig kidney into a human being.
"And that’s really never been done before," she said.
There have recently been developments in the xenotransplant world, but every new step is important. In October, it was announced that researchers at NYU Langone Health had performed a similar experiment to place one pig kidney into a brain-dead human patient.
And this month, surgeons in a Maryland hospital placed a pig heart into a living human patient in an attempt to save his life. He is still being monitored to see how he does.
UAB developed its own cross-match test to see if the pig donor and Parsons would be compatible enough for a transplant, something that is crucial for moving forward in transplanting a pig kidney in a living human.
"Because you can imagine no one’s going to go for taking a kidney if they don’t know up front whether or not it’s going to be rejected," Locke said. "So having that cross-match was critically important."
Pigs have long been considered a possibility for organ transplant in humans because their organs are of a comparable size. It was only recently that scientists surpassed the hurdle of pig organs being rejected when placed in a human environment.
The pigs used for the study had been genetically modified in order to produce kidneys that were able to be used in a human. Crucially, several important human genes had been added, while three pig growth carbohydrate antigens and the pig growth hormone receptor gene had been removed. These specific pigs also didn’t express red blood cell antigens and could serve as universal donors when it comes to blood type.
Although pig organs had been tested for transplant in non-human primates before, this was one of the first times that scientists could test if a pig kidney could function in a human body.
"Non-human primates don’t have the same mean arterial pressure," she explained. "Neither do pigs. We didn’t know if the vascular integrity would hold up, for example."
Using a brain-dead recipient is a unique way to test safety without enrolling a living human being in a clinical trial that could have ended disastrously.
"We were able to approach the family about ouxjmtzywr study, and to say that they were remarkable is an understatement," Locke said.
She also said that "Mr. Parsons and his family allowed us to replicate precisely how we would perform this transplant in a living human. Their powerful contribution will save thousands of lives, and that could begin in the very near future."
In publishing their detailed study about the process, they’ve also accomplished a first by establishing a preclinical human model, Locke said, which essentially means that it sets the precedent for potentially testing new medical advancements in brain-dead donors whose family consents.
"This human preclinical model is a way to evaluate the safety and feasibility of the pig-to-non-human primate model, without risk to a living human," Locke said.
"Our study demonstrates that major barriers to human xenotransplantation have been surmounted, identifies where new knowledge is needed to optimize xenotransplantation outcomes in humans, and lays the foundation for the establishment of a novel preclinical human model for further study."
RELATED IMAGESview larger image

The surgical team prepares the abdomen of the recipient for xenotransplantation. (Jeff Myers/UAB)